Lime softening, also known as Clark's process,[1] is a type of water treatment used for water softening which uses the addition of limewater (calcium hydroxide) to remove hardness (calcium and magnesium) ions by precipitation. The process is also effective at removing a variety of microorganisms and dissolved organic matter by flocculation.[2]
History[edit]
Excess lime and the absence of alkalinity caused by carbonates. The plateau between 60% and 90% of lime is due to the presence of extra alkalinity. Any excess lime added in this region converts bicarbonates to carbonates and those carbonates combine with the free calcium ions to form precipitates. Alkalinity decreased to a minimum at approximately 90% of lime and increased thereafter. This is due to the addition of lime and the precipitation of species, which consumes alkalinity that causes the initial decrease. At lime dosages higher than 120%, alkalinity increases due to an addition of excess lime. For every addition of lime, free. This article is about lime soda water softening. Lime Soda Water Softening Background. Lime soda softening uses addition of lime and soda ash to remove Ca and Mg ions, bringing their concentration down to an acceptable level. The lime may be in the form of quicklime (CaO) or hydrated lime (Ca(OH) 2), also called slaked.
Feb 12, 2018 Microsoft SideWinder Precision Racing Wheel Review. Sep 24, 2019 How i can download driver for microsoft sidewinder precision racing wheel for windows xp / Vista This thread is locked. You can follow the question. 36 rows Microsoft Sidewinder Precision Racing Wheel Driver for Windows 7 32 bit, Windows 7 64 bit, Windows 10, 8, XP. Uploaded on 3/28/2019, downloaded 4932 times, receiving a. Microsoft sidewinder precision racing wheel software download.
Lime softening was first used in 1841 to treat Thames River water. The process expanded in use as the bactericidal effect of the process was discovered. Lime softening greatly expanded in use during the early 1900s as industrial water use expanded. Lime softening provides soft water that can, in some cases, be used more effectively for heat transfer and various other industrial uses.
Chemistry[edit]
As lime in the form of limewater is added to raw water, the pH is raised and the equilibrium of carbonate species in the water is shifted. Dissolved carbon dioxide (CO2) is changed into bicarbonate (HCO−
3) and then carbonate (CO2-
3). This action causes calcium carbonate to precipitate due to exceeding the solubility product. Additionally, magnesium can be precipitated as magnesium hydroxide in a double displacement reaction.[3]
In the process both the calcium (and to an extent magnesium) in the raw water as well as the calcium added with the lime are precipitated. This is in contrast to ion exchange softening where sodium is exchanged for calcium and magnesium ions. In lime softening, there is a substantial reduction in total dissolved solids (TDS) whereas in ion exchange softening (sometimes referred to as zeolite softening), there is no significant change in the level of TDS.
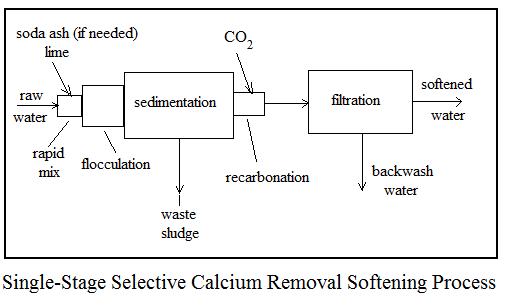
Lime softening can also be used to remove iron, manganese, radium and arsenic from water.
It will also be possible to re-create or repair boot fillers and also change the boot drive. These boot entries can easily be added from any external devices. https://etlucky.netlify.app/easybcd-22-free-download.html. This tool will let the user take full control over the computer’s boot menu and also customize some boot options. The Useful Utilities section will display several options.EasyBCD is an overall powerful modification tool which can come in handy for a user who decides to use it out.
Future uses[edit]
Lime softening is now often combined with newer membrane processes to reduce waste streams. Lime softening can be applied to the concentrate (or reject stream) of membrane processes, thereby providing a stream of substantially reduced hardness (and thus TDS), that may be used in the finished stream. Also, in cases with very hard source water (often the case in Midwestern USA ethanol production plants), lime softening can be used to pre-treat the membrane feed water.
Waste products[edit]
Lime softening produces large volumes of a mixture of calcium carbonate and magnesium hydroxide in a very finely divided white precipitate which may also contain some organic matter flocculated out of the raw water. Processing or disposal of this material may be a cost to the process.
Lime Softening Calculations
References[edit]
- ^Mellor, J W, Intermediate Inorganic Chemistry, Longmans, Green & Co, London, 1941, p. 202
- ^USBR - Lime Softening fact sheetArchived 2011-06-13 at the Wayback Machine
- ^'Lime Softening'. Retrieved 4 November 2011.